Carle June Newsletter 2021
June 15, 2021Continued Gratitude
It’s hard to believe it’s already June. With summer officially arriving and the year reaching its midpoint, we simply wanted to again express our deepest gratitude for all you do to keep our members healthy. The past 15 months have been anything but normal, and yet you’ve continued to provide top quality care and service for the families in our communities. Thank you. Your tireless work – and your dedicated partnership – inspire us.
As It Relates to You
Updates to Our Provider Manuals
We’re busy making updates to our Commercial and Medicare Provider Manuals, and we’re thrilled to announce they’ll be better – and more helpful – than ever. They’ll be released on July 1, and we hope you enjoy the changes we’ve made to address your needs. As always, you’ll be able to find the manuals on our Provider Portal here.
In the updated manuals, you’ll find more information about:
- Our Quality Improvement program – including its purpose, goals, objectives, scope, structure, key personnel, and technical resources and systems.
- Our Care Coordination program – and how to refer your patients.
- How to access and request our utilization management (UM) and pharmaceutical management criteria.
- When decision timeframes begin for non-urgent preservice requests we receive after business hours.
- How to use our pharmacy management procedures and processes – including our formulary, pharmaceutical classes, tier information, prior authorization, managed dose limitations, step therapy, generic substitution, pharmacy savings programs and information on how to request a medical exception.
- Our disease management programs – including what they entail and how you and your patients can access the programs’ services.
- Preventive care guidelines for all age groups.
- Members’ rights and responsibilities..
Additionally, we’d like to remind you of the many resources we have available online on our Provider Portal. Here you’ll find:
- The Compliance Attestation Form, which must be completed on an annual basis in order to comply with guidelines from the Centers for Medicare and Medicaid Services.
- Prospective Provider Forms, as well as Provider Addition and Information Change Forms.
- Credentialing Applications and required forms.
- Prior Authorization Reference Checklists for:
- Concurrent Review (CCR).
- eviCore.
- Outpatient.
- Post Acute.
- Transplant (Admissions).
- Transplant Center.
- Substitution Code Crosswalk Table.
- Change Healthcare ePayment information.
- Our Digital Tools and Guides section, which includes helpful overviews – made just for providers and office personnel – of our prior authorization procedures and of our Provider Portal.
Keeping Your Provider Information Up to Date
Federal and state governments require that providers review and update their information in a timely manner or whenever there are significant changes.
Send all provider updates to provider.updates@HealthAlliance.org. Please note that this is a new email address. Your provider relations specialist will continue to be your contact for all other inquiries. Thank you for all you do.
The “Big Six” – New Videos Made Just for You
We’re excited to bring you six new short videos, giving you a one- to two-minute rundown of coding tips you need to know for each of the “Big Six.” Our risk adjustment (RA) coding consultants partnered with our medical director and RA physician champion, Charles Liang, DO, to create this new video series. Watch here.
The “Big Six” are six diagnosis categories that are often coded incorrectly or with incomplete supporting documentation:
- Vascular Disease.
- Specified Heart Arrhythmias.
- Diabetes Without Complications.
- Diabetes With Complications.
- Chronic Obstructive Pulmonary Disease.
- Congestive Heart Failure.
Each video is short and succinct – perfect for busy providers – and gives you the information you need on how to correctly document and code each of the “Big Six” condition categories to the highest specificity. The videos are just one of many resources we have for you on the Coding Counts section of our Provider Portal.
Good coding is important and keeps your patients healthy. Don’t forget the “Big Six.”
Two Noteworthy Benefit Changes
We’re implementing two noteworthy benefit changes. The U.S. Preventive Services Task Force (Task Force) has recommended starting colorectal cancer screenings at age 45 for average-risk asymptomatic individuals.
In addition, the Task Force has recommended starting Low Dose CT for Lung Cancer Screening at age 50 for individuals with a 20 pack-year smoking history who haven’t quit in the last 15 years.
We’ll be adjudicating these two benefits as wellness, without member cost share, beginning July 1, 2021, for our fully insured, non-transitional individual and group plans, excluding short-term, limited-duration plans. Many of our self-funded plan sponsors will mirror this coverage, although the timing may vary for certain self-funded plans. Please note – these July 1 changes do not impact benefits or coverage for our Medicare Advantage plans.
If you have any questions, please contact your provider relations specialist.
New CPT Code for Low Dose CT for Lung Cancer Screening
As of January 1, 2021, the CPT code G0297 has been deleted and replaced with new code 71271 for Low Dose CT for Lung Cancer Screening. Our claims system has already been updated to reflect this.
Help Us Move the Needle
National Men’s Health Month
June is National Men’s Health Month – the perfect time to remind all men (and boys) to stay on top of their health by eating right, exercising and keeping up with their regular doctor visits and checkups. Encourage all the men in your lives to:
- Eat healthy – add more fruits and vegetables into their diet, and limit foods high in calories, sugar, salt and fat.
- Get moving – make a personal goal to reach 2.5 hours of physical activity per week. To stay motivated, they should do a sport or activity they enjoy.
- Quit tobacco – smoking tobacco is the number one preventable cause of death in the U.S., and the primary cause of both COPD and lung cancer.
- Make prevention a priority – schedule yearly checkups and regular health screenings:
- Annual physical exams to review overall health status. Each year, they should get a thorough physical exam and discuss health-related topics – including blood pressure and cholesterol – with their doctor.
- Shingles vaccine if 50 and older. This is especially important for those with COPD or diabetes, as these are high-risk conditions for shingles.
- Osteoporosis prevention if 45 and older, and at higher risk for osteoporosis (because of low body weight, smoking, alcohol use, older age, or family history of osteoporosis or hip fracture).
- Colon cancer screening if 45 and older. Options include:
- FIT testing annually.
- Cologuard® every three years.
- Sigmoidoscopy every five years.
- Colonoscopy every 10 years.
Also encourage the men you know to set an example for their sons, brothers, co-workers and the other men and boys around them. By following the above tips, they can be a role model for those who may be watching. Every person can make a difference for the health of others.
Diabetes Preventive Screenings
We’ve mentioned it many times, but it’s worth repeating – preventive healthcare screenings are essential for people with diabetes. National guidelines lay out a number of screenings that need to be completed to help prevent complications of the disease.
Unfortunately, a recent study found that fewer than 60% of patients with diabetes got their recommended screenings – 58% for HbA1C tests and 57% for lipid profile tests.
Pre-visit screenings can increase the likelihood that your patients with diabetes get their appropriate preventive tests. We also recommend using a template like the one below, which can further improve the chances that your patients complete all their screenings. Together we can help those with diabetes live their healthiest lives.
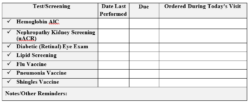
Yearly Nephropathy Screenings for Patients with Diabetes
Did you know that 20 to 40% of people diagnosed with diabetes also develop kidney disease? Or that 40 to 50% of all diagnosed cases of end-stage renal disease (ESRD) are related to diabetes? Despite these alarming stats, many people with diabetes do not get their recommended yearly nephropathy urine screenings. As providers, you can help ensure your patients stay up to date with this vital screening, so kidney damage can be detected early and interventions can be started if needed.
For all of your patients with diabetes, please remember to:
- Review their health record and order a nephropathy screening if it’s due. If it’s not due yet, give them a reminder of when it’ll be due.
- Educate them about how symptoms of kidney failure usually don’t occur until nearly all function is lost, which is why yearly nephropathy screenings are so important to their health.
- Remind them to stay within their target blood sugar ranges and keep their blood pressure controlled.
- Encourage them to lead a healthier lifestyle, and to engage in health coaching or care coordination. Our members can call the number on the back of their member ID card or visit the webpage linked above to find out more about these helpful services.
Finally, please see – and make use of – this Kidney Health Toolkit from the National Committee for Quality Assurance. The downloadable Education for Providers gives a quick and helpful overview of how to assess for, monitor and manage kidney disease in your patients – including useful information on how to interpret test results from nephropathy urine screenings.
Thank you – we deeply appreciate all you do to help keep our members healthy.
Help Increase Vaccinations for Shingles
Shingles (herpes zoster) is a disease caused by the reactivation of the varicella zoster virus, the same virus that causes chicken pox. Shingles is characterized by a painful rash and the most common complication – in spite of treatment – is chronic pain in the affected area (post-herpetic neuralgia). Cases of shingles increase sharply with age, being more common in those over 50 and affecting up to 50% of people 85 and older. Along with age, underlying diseases like diabetes and chronic obstructive pulmonary disease (COPD) also increase the risk of developing shingles, along with its severity and impact.
The Centers for Disease Control and Prevention (CDC) estimates that 25 to 33% of adults will get shingles at some point in their life. Of these, one-third will develop complications – most commonly nerve pain that lasts for months or even years after the rash heals.
To prevent shingles – and its related complications – the CDC recommends the vaccine called SHINGRIX (recombinant zoster vaccine). They recommend two doses of SHINGRIX, separated by two to six months, for immunocompetent adults age 50 and older.
SHINGRIX is recommended:
- Whether or not someone reports a prior episode of shingles.
- Whether or not someone reports a prior dose of ZOSTAVAX® (a shingles vaccine no longer available in the U.S.).
And, to answer common FAQs, the vaccine can indeed still be given to those who:
- Have chronic medical conditions – such as chronic renal failure, diabetes mellitus, rheumatoid arthritis or chronic obstructive pulmonary disease (COPD) – unless a contraindication or precaution exists (see below).
- Are taking low-dose immunosuppressive therapy.
- Are anticipating immunosuppression.
- Have recovered from an immunocompromising illness.
- Are getting other adult vaccines – such as flu and pneumococcal shots – in the same doctor visit.
Additionally, please note that it’s not necessary to screen, either verbally or by laboratory serology, for evidence of prior varicella infection. For more detailed information on the CDC’s recommendations, visit this webpage.
Contraindications and Precautions for Shingles Vaccination
- A history of severe allergic reaction – such as anaphylaxis – to any component of the vaccine or to a previous dose of the vaccine.
- A person who’s known to be seronegative for varicella.
It’s not necessary to screen (either verbally or by laboratory serology) for a history of varicella. However, if a person is known to be varicella-negative via serologic testing, you should follow ACIP guidelines for varicella vaccination. - A person experiencing an acute episode of shingles.
The vaccine is not a treatment for shingles or post-herpetic neuralgia. - Adults with a minor acute illness, such as a cold, can receive SHINGRIX. However, those with a moderate or severe acute illness should usually wait until they recover to get the vaccine. This includes anyone with a temperature of 101.3°F or higher.
- Providers should consider delaying vaccination for pregnant women and those who are breastfeeding.
To learn much more about the contraindications and precautions, please visit this CDC webpage.
Vaccine Coverage Information
We cover SHINGRIX as preventive on our commercial plans for those age 50 and above. On our Medicare plans, it’s covered on Tier 3 on the Part D benefit.
Other sources used for this article:
ncbi.nlm.nih.gov/pmc/articles/PMC5934818 ncbi.nlm.nih.gov/pmc/articles/PMC6104256
Metabolic Monitoring Toolkit
The National Committee for Quality Assurance (NCQA) is the credentialing body for our health plans. Each year, the NCQA releases Healthcare Effectiveness Data and Information Set (HEDIS®) standards that we use to guide our quality measures. Many of the standards focus on behavioral health.
This year, our Quality department has created a toolkit we’d like to roll out to promote greater adherence to some of these behavioral health standards. Here’s an example from the toolkit, for one of these standards:

The “Measure Description” section in each toolkit entry provides a short summary of that particular HEDIS standard. The standard above seeks to promote metabolic monitoring for children and adolescents who are prescribed antipsychotic medications. The “Measure Importance” section explains why the particular standard is significant. In the example above, it’s because antipsychotic medications are known to cause metabolic syndrome, which can result in poor cardiovascular outcomes. The final section lists what you – the provider – can do to support this standard and keep the patient healthy.
If you have any questions or would like to know more about our new toolkit for these behavioral health standards, please contact your provider relations specialist and have them connect you to our Quality department. We’re always happy to give you the resources you need as you help keep our members healthy and well.
HEDIS ADHD Quality Measure Overview
As the previous article mentions, we use HEDIS standards to guide our quality measures and drive our improvement efforts. This article provides an overview of the HEDIS measure for attention-deficit/hyperactivity disorder (ADHD).
Description of What’s Measured
The percentage of children newly prescribed ADHD medication who had at least three follow-up care visits within a 10-month period, one of which was within 30 days of when the first ADHD medication was dispensed. Two rates are reported:
- Initiation Phase rate. The percentage of children 6 – 12 years old with an ambulatory prescription dispensed for ADHD medication, who had one follow-up visit with a practitioner with prescribing authority during the 30 days following the prescription dispense date.
- Continuation and Maintenance Phase rate. The percentage of children 6 – 12 years old with an ambulatory prescription dispensed for ADHD medication, who remained on the medication for at least 210 days and who – in addition to the visit in the Initiation Phase – had at least two more follow-up visits with a practitioner within 270 days (nine months) after the Initiation Phase ended.*
*Please note that telehealth video and phone appointments are now available for the second and third follow-up appointments.
Measure Inclusion Criteria
- Children age 6 – 12 who are dispensed an ADHD medication, as long as they hadn’t received one in the 120 days prior.
When does someone ‘pass’ the measure?
- Initiation Phase: When they’ve had an in-person visit with a practitioner with prescribing authority within 30 days of the prescription being dispensed.
- Continuation and Maintenance Phase: When they’re compliant in the Initiation Phase and have had at least two more follow-up visits – on different dates – with any practitioner, between days 31 and 300 following the prescription being dispensed.
Strategies for Improvement
- If you prescribe a medication used for ADHD, consider limiting the first prescription to a 30-day supply.
- Educate the parent or guardian that the child must be seen within 30 days of starting the medication, to evaluate if it’s working as expected and to assess any adverse effects.
- Use telehealth video and phone appointments for the second and third follow-up appointments, if they’re more convenient for you and the patient.
Sources used for this article:
- org/hedis/measures/follow-up-care-for-children-prescribed-adhd-medication
- com/pennsylvania/assets/pdf/provider/notices/quality-improvement/2019%20FollowUp%20Care%20-%20Children%20-%20ADHD%20Meds%20ADD%2018179.pdf
- com/wp-content/uploads/HEDIS-ADD-Provider-Tip-Sheet.pdf
Opioid Prescribing Guidelines from the CDC
The Centers for Disease Control and Prevention (CDC) has developed guidelines for prescribing opioid medications in the primary care setting, for those 18 and older who have chronic pain. These guidelines do not apply to those involved in palliative care, cancer treatments and end-of-life care.
Opioids are not routine therapy. Nor are they first-line treatment for chronic pain. The CDC guidelines are as follows:
- Determine when to start opioids for chronic pain.
- What are the treatment goals?
- Realistic goals for function and pain.
- How to discontinue if non-therapeutic.
- Discuss the benefits and risks of opioid therapy with the patient.
- Discuss non-pharmacologic therapy options with the patient.
- These are preferred for chronic pain.
- If opioids are used, it should be in combination with non-pharmacologic therapy.
- Opioid selection, duration, dosage, follow up and discontinuation.
- Consideration of dosage: start low, go slow.
- Discuss duration of treatment.
- For acute pain, three days is usually sufficient and more than seven days is rare.
- Selection of immediate-release vs. extended-release/long-acting opioids:
- Use immediate-release when starting.
- Discontinuation and follow up (of therapy with opioids):
- Evaluate harms/benefits within one to four weeks after starting.
- If continued therapy, reevaluate every three months or sooner.
- Assessing harms and risks of opioid use.
- Benzodiazepine co-prescription: avoid.
- Treatment for opioid use disorder:
- Discussion of Medication Assisted Therapy (MAT).
- Buprenorphine or methadone with behavioral therapy.
- Evaluation of risks/mitigation of risks.
- Prescription drug monitoring program (PDMP) data review:
- Review for high dosages and multiple providers.
- Urine drug testing possibility:
- Before beginning opioid therapy and at least annually thereafter.
You can see the full guidelines by visiting this CDC webpage.
Help Your Patients Quit Smoking
Did you know that most of our health plans cover smoking cessation medications and coaching for our members?
Our smoking and vaping cessation program, Quit For Life®, is a great first step. Quit For Life is the nation’s leading tobacco cessation program, complete with one-on-one support from a Quit Coach®. Here are some more details:
- Members can enroll once every 12 months (equals two quit attempts).
- They can use a combination of nicotine replacement therapy (NRT) and Zyban®, or Chantix®. There’s no pharmaceutical copay if accompanied by a prescription.
- They can get patches, gum and lozenges through our own pharmacy without a prescription, when we mail directly to them.
And they also can get:
- Behavioral support that lasts six months.
- Help understanding their medication.
- One-on-one coaching by phone.
- A personal quit plan made just for them.
Tobacco harms far too many lives. We ask for your help in encouraging your patients to get the support they need. Let them know about this covered program, and point them to this webpage to learn more. Together we can help them Quit For Life.
Pharmacy 101: How We Choose New Drugs for Formularies
Providers often ask us how we choose new medications for our formularies. Like with all else we do, we follow best practices and adhere to strict policies, keeping the well-being of our members foremost. Here are the basics of our process.
We have a Pharmacy and Therapeutics committee made up of voting members (one of our own medical directors, one of our pharmacists and representative physicians and pharmacists from our networks) and non-voting subcommittee members (physician specialists and pharmacists). This committee is responsible for establishing and maintaining our formularies.
The committee meets every other month to review and approve changes to our formularies. These include adding drugs, removing drugs and tier changes. When considering additions, the committee reviews drugs new to the market within six months of their product launch – and in most cases sooner. They evaluate the drugs and also current drug policies to make sure best practice standards are being followed. As a result of this evaluation, they may place the new drug(s) on our formularies (a positive change).
In cases of removing a drug from the formulary (a negative change), the committee does this twice yearly (January 1 and July 1). Whenever a drug is removed, we notify affected members – via first-class mail – 60 days before we make the change to our formulary. Altogether, both positive and negative changes account for a less than 1% annual change in our formularies. Our members can always log into their account at hally.com or use the Hally® mobile app to view the most up-to-date drug lists. And we encourage our providers to reach out to us if you have any questions about formularies or other pharmacy practices and policies. We’re always happy to help.
Pharmacy 101: Why Some Drugs Aren’t Covered
You may also wonder why our health plans don’t cover certain medications. We again follow strict guidelines and procedures, and there are multiple reasons why we exclude certain drugs from coverage.
Our Pharmacy and Therapeutics committee maintains an Excluded Drug List. This is a listing of drugs not covered under our pharmacy benefit. Drugs often placed on the list include:
- Formulations of drugs (brand or generic) that have a commercially available generic drug in the same therapeutic class, and that offer no additional clinical value – as demonstrated in peer review literature – over the current existing therapies in the treatment class.
- Formulations of drugs (brand or generic) that are a combination of two or more existing drugs (prescription or over-the-counter), that offer no additional clinical benefit compared to taking the individual drugs together.
- Brand-name formulations of drugs used in the dermatology space (oral and topical) that have an existing covered generic product and offer no additional clinical benefit (cosmetic pleasing formulations).
- Prescription-strength benzoyl peroxide and combination products.
- Drugs not considered medically necessary, including but not limited to: drugs used for cosmetic use, weight loss medications and drugs used to treat decreased sexual desire.
- Non-prescription (over-the-counter) drugs, with the exception of insulin, insulin syringes and items required for coverage as part of Preventive Health and/or Women’s Wellness Benefits.
- Non-sedating antihistamines and combinations with an over-the-counter alternative.
- Products classified as Prescription Medical Devices by the Food and Drug Administration.
- Products classified as Medical Food or supplements.
- Drugs that haven’t been approved as effective by the Food and Drug Administration, including DESI drugs.
You can find the Excluded Drug List policy here. Please note that this policy applies to all plans (not just Health Alliance Northwest plans), excluding our Medicare plans.
Pharmacy Updates
All Plans
Oncology and Infectious Disease Updates
Formulary Additions
- Cabenuva (cabotegravir-rilpivirine) and Vocabria (cabotegravir)—Treatment of HIV-1 infection in adults
- Formulary placement recommendations
- Commercial—Non-Preferred Specialty Medical
- Medicare—Tier 5 Specialty Pharmacy, Part B vs. D determination for Cabenuva
- Formulary placement recommendations
- Tepmetko (tepotinib—Treatment of metastatic non–small cell lung cancer in adults harboring mesenchymal-epithelial transition (MET) exon 14 skipping alterations
- Formulary placement recommendations
- Commercial—Non-Preferred Specialty Pharmacy with PA; reviewed by eviCore when applicable
- Medicare—Tier 5 with PA; reviewed by Health Alliance
- Formulary placement recommendations
- Ukoniq (umbralisib)—(1) Treatment of relapsed or refractory follicular lymphoma in adults who have received at least three prior lines of systemic therapy. (2) Treatment of relapsed or refractory marginal zone lymphoma in adults who have received at least one prior anti-CD20-based regimen
- Formulary placement recommendations
- Commercial—Non-Preferred Specialty Pharmacy with PA; reviewed by eviCore when applicable
- Medicare—Tier 5 with PA; reviewed by Health Alliance
- Formulary placement recommendations
- Fotivda (tivozanib)—Treatment of relapsed or refractory advanced renal cell carcinoma (RCC) in adults following ≥2 prior systemic therapies
- Formulary placement recommendations
- Commercial—Non-Preferred Specialty Pharmacy with PA; reviewed by eviCore when applicable
- Medicare—Tier 5 with PA; reviewed by Health Alliance
- Formulary placement recommendations
- Pepaxto (melphalan flufenamide)—Treatment of relapsed or refractory multiple myeloma (in combination with dexamethasone) in adults who have received at least four prior lines of therapy and whose disease is refractory to at least one proteasome inhibitor, one immunomodulatory agent and one CD38-directed monoclonal antibody.
- Formulary placement recommendations
- Commercial—Non-Preferred Specialty Medical with PA; reviewed by eviCore when applicable
- Medicare—Part B; reviewed by eviCore
- Formulary placement recommendations
- Breyanzi (lisocabtagene maraleucel)—Treatment of relapsed or refractory large B-cell lymphoma in adults after ≥ 2 lines of systemic therapy, including diffuse large B-cell lymphoma not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma and follicular lymphoma grade 3B
- Formulary placement recommendations
- Commercial—Non-Preferred Specialty Medical with PA; reviewed by eviCore when applicable
- Medicare—Part B; reviewed by eviCore
- Formulary placement recommendations
- Margenza (margetuximab)—Treatment of metastatic HER2-positive breast cancer (in combination with chemotherapy) in adults who have received two or more prior anti-HER2 regimens, at least one of which was for metastatic disease
- Formulary placement recommendations
- Commercial—Non-Preferred Specialty Medical with PA; reviewed by eviCore when applicable
- Medicare—Part B; reviewed by eviCore
- Formulary placement recommendations
Dermatology
Formulary Additions
- Klisyri (tirbanibulin)—Treatment of actinic keratosis on the face or scalp
- Formulary placement recommendations
- Commercial—Non-Preferred Brand with PA
- Medicare—Non-Formulary
- Formulary placement recommendations
- Scenesse (afamelanotide)—Indicated to increase pain-free light exposure in adult patients with a history of phototoxic reactions from erythropoietic protoporphyria (EPP)
- Formulary placement recommendations
- Commercial—Tier 6 with PA
- Medicare—Part B with PA
- Formulary placement recommendations
- Winlevi (clascoterone)—Treatment of acne vulgaris in patients 12 years of age and older
- Formulary placement recommendations
- Commercial—Non-Preferred Brand with PA
- Medicare—Non-Formulary
- Formulary placement recommendations
Rheumatology
Formulary Additions
- Lupkynis (voclosporin)—Treatment of active lupus nephritis in combination with mycophenolate mofetil and corticosteroids
- Formulary placement recommendations
- Commercial—Non-Preferred Specialty Pharmacy with PA
- Medicare—Tier 5 Specialty Pharmacy with PA
- Formulary placement recommendations
Commercial
Tier Changes
Commercial Tier Changes
- Bonjesta: Move from Non-Preferred Brand to Excluded
- Branded doxylamine/pyridoxine product
- Recommend excluding since individual ingredients can be purchased over the counter
Criteria Changes
- Nplate (romiplostim)
- Removed step through Promacta and updated references
- Sandostatin (octreotide) and Sandostatin LAR (octreotide)
- Added Bynfezia pen to policy
Gastroenterology
Criteria Changes
- Gattex (teduglutide)
- Updated approval period section to require criteria for subsequent approvals and updated references
- Xifaxan (rifaximin)
- Decreased prerequisite therapy requirements from four agents to three agents for IBS-D and updated references
Criteria Changes
- Polyarticular Juvenile Idiopathic Arthritis Immunomodulator Therapies
- Added Xeljanz (Preferred with single step through Humira) and Simponi Aria (Preferred) to policy
- This changes Enbrel and Kineret to requiring a quadruple step-edit
- Kineret (anakinra)
- Added coverage criteria for DIRA and AOSD
- Ilaris (canakinumab)
- Added coverage criteria for AOSD