Midwest October Newsletter
October 19, 20172017 Provider and Risk Adjustment Workshop
We’re partnering with Carle to present a provider workshop on Thursday, October 26 at the Carle Forum. Doors open at 5:30 p.m., and the workshop starts at 6 p.m.
Our 3 speakers will include:
- Dr. James Leonard, President and CEO of Carle, talking about the effects and advantages of risk adjustment for Carle and Health Alliance.
- Dr. Robert Good, Chief Medical Officer, covering population health.
- Dr. Jens Yambert, Medical Director Risk Adjustment Revenue Management, discussing the provider education module available for 2018.
CMS Education on Medicare Skilled Nursing and Therapy Services Coverage
The Centers for Medicare and Medicaid (CMS) want to remind you that the Jimmo v. Sebelius Settlement Agreement clarified that Medicare covers skilled nursing care and skilled therapy services under its skilled nursing facility, home health, and outpatient therapy benefits when a patient needs skilled care in order to maintain function or to prevent or slow decline or deterioration (provided all other coverage criteria are met). This may reflect a change in practice for those of you who thought that Medicare only covers nursing and therapy services under these benefits when a patient is expected to improve.
Specifically, this settlement required manual revisions to restate a maintenance coverage standard for both skilled nursing and therapy services under these benefits:
- Skilled nursing services would be covered where such services are necessary to maintain the patient’s current condition or prevent or slow further deterioration, so long as the patient requires skilled care for the services to be safely and effectively provided.
- Skilled therapy services are covered when an individualized assessment of the patient’s clinical condition demonstrates that the specialized judgment, knowledge, and skills of a qualified therapist are necessary for the performance of a safe and effective maintenance program. This type of maintenance program to maintain the patient’s current condition or to prevent or slow further deterioration is covered so long as the patient requires skilled care for the safe and effective performance of the program.
This decision does not:
- Mandate that you use daily services over intermittent skilled services for maintenance therapy.
- Override benefit limits. Benefit limits are still in place for certain services, which may prevent indefinite treatment in some situations.
- Require observation and assessment by a nurse for the treatment of the illness or injury when the characteristics are part of a longstanding pattern of a waxing and waning condition, which by themselves don’t require skilled services, and when there’s no attempt to change the treatment to resolve them. (A3-3132.1.C.2, SNF-214.1.C.2)
As part of our educational efforts to make sure services are provided and coverage determinations are adjudicated accurately and in accordance with existing Medicare policy, we ask that you review this educational information from CMS:
- New clarified summary
- CMS Manual Updates to Clarify Skilled Nursing Facility (SNF), Inpatient Rehabilitation Facility (IRF), Home Health (HH), and Outpatient (OPT) Coverage Pursuant to Jimmo vs. Sebelius
- MLN Matters on Manual Updates to Clarify Skilled Nursing Facility (SNF), Inpatient Rehabilitation Facility (IRF), Home Health (HH), and Outpatient (OPT) Coverage Pursuant to Jimmo vs. Sebelius
- Jimmo v. Sebelius Settlement Agreement Fact Sheet
- Jimmo v. Sebelius Settlement Agreement Program Manual Clarifications Fact Sheet
- PowerPoint from the National Call for Contractors and Adjudicators – December 16, 2013
eviCore Online Clinical Consultation Scheduling
eviCore is excited to introduce their online clinical consultation scheduling system to help streamline the process for requesting peer-to-peer consultations.
You can schedule clinical consultations for our members’ cases faster on eviCore.com. You can also find this from the eviCore.com provider login page, beneath the log in button.

Note: You can’t access this feature from Your Health Alliance for providers, you must go to eviCore.com. You will have to continue to go to Your Health Alliance for providers to submit eviCore preauthorization request.
From there, choose Health Alliance Med Plan from the Select Health Plan dropdown and select your solution.
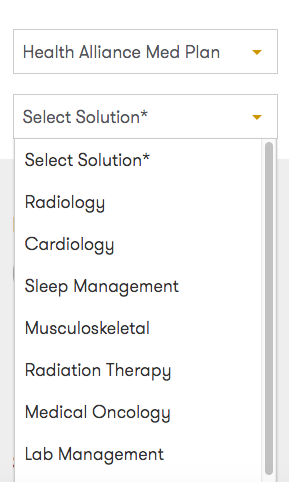
Fill out the form with the required info, and submit. Then you’ll receive a confirmation email, and an agent will contact you before the time in your request to schedule your exact appointment time.
Oncology Preauthorizations on eviCore
For which oncology patients is preauthorization on eviCore through Your Health Alliance for providers required? We can help:
| Patient Type | When Is PA Required? |
|---|---|
| Patients on stand-alone medications who have approvals on file with us for medications that required PA before August 1, 2017 | When the current authorization expires, or if treatment or medication changes. |
| Patients on standalone medications or regimens that did not require PA before August 1, 2017 | Required as of August 1, 2017. |
| Patients on stand-alone medications or regimens who have an approval on file with us for |
When the current authorization expires. We recommend these requests be placed through eviCore if the provider group would like an authorization verifying the full regimen. |
Medicaid Claims
In our December 2016 Newsletter, we reminded all participating providers that we terminated our Medicaid contracts with Healthcare and Family Services, effective December 31, 2016. We asked all participating providers to submit all Medicaid claims with dates of service on or before December 31, 2016, no later than April 1, 2017.
We will not process claims received after that date. This is subject to your contract terms related to timely filing. We appreciate your understanding.
Reminder About In-Network Referrals
Remember to use contracted in-network providers before referring a patient outside our network. You can search a member’s network on Your Health Alliance for providers and office personnel by attaching to that member.
When our members need services that aren’t available from an in-network provider, they might also be able to get those services from a provider in their secondary or tertiary network. You can also access this while attached to a member. Note: All providers in secondary or tertiary networks require preauthorization.
Pharmacy Updates
All Plans
Formulary Additions
- Kevzara (sarilumab) – Indicated for the treatment of adult patients with moderate to severe Rheumatoid Arthritis (RA) who have had an inadequate response or intolerance to one or more biologic or non-biologic Disease-Modifying Anti-Rheumatic Drugs (DMARDs).
- Commercial – Tier 5 with preauthorization (PA)
- Medicare – Non-formulary
- Austedo (deutetrabenazine) – Indicated for the treatment of chorea associated with Huntington’s disease (HD).
- Commercial – Tier 6 with PA
- Medicare – Non-formulary
- Ingrezza (valbenazine) – Indicated for the treatment of tardive dyskinesia (TD).
- Commercial – Tier 5 with PA
- Medicare – Tier 5 with PA
- Ocrevus (ocrelizumab) – Indicated for the treatment of relapsing or primary progressive forms of multiple sclerosis (MS) in adult patients.
- Commercial – Tier 5 with PA
- Medicare – Tier 5 with PA
- Radicava (edaravone) – Indicated for the treatment of Amyotrophic Lateral Sclerosis (ALS).
- Commercial – Tier 6 with PA
- Medicare – Non-formulary
- Xadago (safinamide) – Indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes.
- Commercial – Tier 3
- Medicare – Non-formulary
Formulary Additions – Effective October 4, 2017
- Brineura (cerliponase alfa) – Indicated to slow the loss of ambulation in symptomatic pediatric patients 3 years of age and older with late infantile neuronal ceroid lipofuscinosis type 2 (CLN2), also known as tripeptidyl peptidase 1 (TPP1) deficiency.
- Commercial – Tier 6 with PA
- Medicare – Part B only
- Emflaza (deflazacort) – Indicated for the treatment of Duchenne muscular dystrophy in patients 5 years of age and older.
- Commercial – Tier 6 with PA
- Medicare – Non-formulary
- Spinraza (nusinersen) – Indicated for the treatment of spinal muscular atrophy (SMA).
- Commercial – Tier 6 with PA
- Medicare – Part B only
- Tremfya (guselkumab) – Indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
- Commercial – Tier 5 with PA
- Medicare – Non-formulary
- Tymlos (abaloparatide) – Indicated for the treatment of postmenopausal women with osteoporosis at high risk for fracture defined as a history of osteoporotic fracture, multiple risk factors for fracture, or patients who have failed or are intolerant to other available osteoporosis therapy.
- Commercial – Tier 5 with PA
- Medicare – Non-formulary
- Haegarda (C1 inhibitor [human]) – Indicated as routine prophylaxis against angioedema attacks in adults and adolescents with hereditary angioedema.
- Commercial – Tier 6 with PA
- Medicare – Tier 6 with PA
- Nityr (nitisinone) – Indicated for the treatment of hereditary tyrosinemia type 1.
- Commercial – Tier 6 with PA
- Medicare – Non-formulary
- Hepatitis C
- Mavyret (glecaprevir and pibrentasvir) – Indicated for the treatment of chronic hepatitis C virus genotype 1, 2, 3, 4, 5, or 6 infection in adults without cirrhosis or with compensated cirrhosis, HCV genotype 1 infection in adults previously treated with a regimen containing an HCV NS5A inhibitor, or an NS3/4A protease inhibitor, but not both.
- Commercial – Tier 4 with PA
- Medicare – Tier 5 with PA
- Vosevi (sofosbuvir, velpatasvir, and voxilaprevir) – Indicated for the treatment of adults with chronic hepatitis C virus (HCV) infection, without cirrhosis or with compensated cirrhosis (Child-Pugh class A), who have genotype 1, 2, 3, 4, 5, or 6 infection, and have previously been treated with an HCV regimen containing an NS5A inhibitor, or who have genotype 1a or 3 infection and have previously been treated with an HCV regimen containing sofosbuvir without an NS5A inhibitor.
- Commercial – Tier 6 with PA
- Medicare – Non-formulary
- Mavyret (glecaprevir and pibrentasvir) – Indicated for the treatment of chronic hepatitis C virus genotype 1, 2, 3, 4, 5, or 6 infection in adults without cirrhosis or with compensated cirrhosis, HCV genotype 1 infection in adults previously treated with a regimen containing an HCV NS5A inhibitor, or an NS3/4A protease inhibitor, but not both.
- Oncology
- Kisqali (ribociclib) 200mg tablet – Indicated for breast cancer, advanced or metastatic.
- Commercial – Tier 5 with PA reviewed by eviCore
- Medicare – Tier 5 with PA reviewed by Health Alliance
- Nerlynx (neratinib) 40mg tablet – Indicated for breast cancer.
- Commercial – Tier 5 with PA reviewed by eviCore
- Medicare – Tier 5 with PA reviewed by Health Alliance
- Bavencio (avelumab) 200mg/10mL IV – Indicated for Merkel cell carcinoma, metastatic, and urothelial carcinoma, locally advanced or metastatic.
- Commercial – Tier 5 with PA reviewed by eviCore
- Medicare – Tier 5 with PA, reviewed by Health Alliance if covered under Part D, reviewed by eviCore if covered under Part B
- Rydapt (midostaurin) 25mg capsule – Indicated for acute myeloid leukemia, FLT3-positive, mast cell leukemia, and systemic mastocytosis.
- Commercial – Tier 5 with PA reviewed by eviCore
- Medicare – Tier 5 with PA reviewed by Health Alliance
- Alunbrig (brigatinib) 30mg tablet – Indicated for non-small cell lung cancer, metastatic.
- Commercial – Tier 5 with PA reviewed by eviCore
- Medicare – Tier 5 with PA reviewed by Health Alliance
- Imfinzi (durvalumab) 120mg/2.4mL, 500mg/10mL IV – Urothelial carcinoma, locally advanced or metastatic.
- Commercial – Tier 5 with PA reviewed by eviCore
- Medicare – Tier 5 with PA, reviewed by Health Alliance if covered under Part D, reviewed by eviCore if covered under Part B
- Kisqali (ribociclib) 200mg tablet – Indicated for breast cancer, advanced or metastatic.
Commercial
Criteria Changes
- Bunavail, Suboxone, and Zubsolv
- Removed Subutex from policy (brand name is discontinued)
- Added Bunavail and Zubsolv to policy
- Tier 3 with PA
- Pennsaid
- Retired
- Moving to Excluded
- Rheumatology
- Actemra
- Added criteria for Giant Cell Arteritis/Temporal Arteritis – Newer indication
- Documented diagnosis, ordered by a neuro-ophthalmologist, and failure to respond to glucocorticoids
- Update: Dr. Rasheed requested broadening of provider requirement to include ophthalmologists and rheumatologists
- Added criteria for Giant Cell Arteritis/Temporal Arteritis – Newer indication
- Cosentyx
- Updated criteria for Ankylosing Spondylitis
- Removed step through intra articular steroids and through sulfasalazine to align our policy with ACR guidelines
- ACR guidelines indicate that NSAIDs and TNFs are strong first- and second-line recommendations
- Updated criteria for Ankylosing Spondylitis
- Enbrel
- Updated criteria for Plaque Psoriasis
- Age restriction was changed from 18 years or older to 4 years or older, as the FDA label has recently changed
- Updated criteria for Plaque Psoriasis
- Humira
- Added criteria for Uveitis – New indication
- Documented diagnosis, ordered by an ophthalmologist, failure to respond to topical glucocorticoids, and failure to respond to systemic glucocorticoids or immunosuppressive agents
- Update: Dr. Rasheed requested broadening of provider requirement to include ophthalmologists, rheumatologists, and providers experienced in the treatment of uveitis
- Added criteria for Pyoderma Gangrenosum
- Off-label indication, however we currently cover Remicade for this indication, so we would like to add coverage for our preferred product Humira
- Documented diagnosis, ordered by a specialist, and documentation of not responding to standard therapy
- Added criteria for Uveitis – New indication
- Ilaris
- Added criteria for Systemic Juvenile Idiopathic Arthritis
- Documented diagnosis with moderate to severe disease, age 2 or older, ordered by a rheumatologist, failure of one NSAID
- Added criteria for Systemic Juvenile Idiopathic Arthritis
- Orencia
- Added criteria for Psoriatic Arthritis – New indication
- Documented diagnosis, ordered by a rheumatologist, failure on a DMARD, failure on Humira or Enbrel
- Added criteria for Psoriatic Arthritis – New indication
- Remicade
- Added biosimilar Renflexis to policy
- Updated criteria for Ankylosing Spondylitis
- Removed step through intra articular steroids and through sulfasalazine to align our policy with ACR guidelines
- ACR guidelines indicate that NSAIDs and TNFs are strong first- and second-line recommendations
- Updated criteria for Pyoderma Gangrenosum – Off-label
- Added Humira step-therapy (ST) since Humira is now also used for Pyoderma Gangrenosum treatment and is a preferred product
- Rituxan
- Updated criteria for Autoimmune Hemolytic Anemia
- Removed ST through azathioprine or cyclophosphamide
- Removed ST through cyclosporine or mycophenolate
- According to Uptodate, Rituxan is second-line treatment, following corticosteroids
- Updated criteria for Immune Thrombocytopenic Purpura
- Revised criteria to allow coverage if splenectomy is contraindicated
- Updated criteria for Systemic Lupus Erythematosus
- Updated ST through hydroxychloroquine or chloroquine to also check claims history for compliance
- We do this for Benlysta as well
- Updated criteria for Granulomatosis with Polyangitis
- Revised terminology from Wegener’s Granulomatosis to Granulomatosis with Polyangitis
- Removed ST through cyclophosphamide and azathioprine as cyclophosphamide and Rituxan are equally recommended as second-line, and azathioprine is not recommended for organ threatening disease
- Added criteria for Multiple Sclerosis
- This drug is pharmacologically similar to Ocrevus (new monoclonal antibody indicated for treatment of PPMS and RRMS)
- Off label for MS, but is less costly than Ocrevus
- Updated criteria for Autoimmune Hemolytic Anemia
- All Policies
- Updated exclusion criteria to indicate that we will not continue to cover if an inadequate response was achieved
- Updated exclusion criteria to indicate that we will not cover concurrent therapy with multiple biologic DMARDS or other TNF blockers
- Actemra
- Psychiatry
- Behavioral Health
- Updated Brand Name Antidepressants section to Non-Preferred Antidepressants
- This allows generic desvenlafaxine to also follow this criteria, as we still PA this product
- Add Khedezla to Non-Preferred Antidepressant section
- This is also a desvenlafaxine product, and we want to PA it to be consistent with the PA we have on Pristiq
- Updated Brand Name Antidepressants section to Non-Preferred Antidepressants
- Dyanavel XR Suspension, Quillichew ER, Quillivant XR Policies
- Updated age requirement from covered for ages 12 and under to covered for members ages 6 to 12 years, as these products are all indicated for children over age 6
- Behavioral Health
- Neurology
- Provigil and Nuvigil policy
- Retired policy
- Both drugs have generics and prices no longer warrant PA
- Retired policy
- Xyrem
- Updated to require a sleep lab evaluation confirming diagnosis of narcolepsy
- Xyrem policy requires previous failure of modafinil or armodafinil, the use of which currently requires a sleep lab evaluation for PA
- If PA is removed from modafinil and armodafinil, the sleep lab is no longer ensured and Xyrem could process without confirmed diagnosis of narcolepsy
- Drug must be requested by a Xyrem certified REMS provider who is familiar with the risks and adverse effects of Xyrem
- Updated to require a sleep lab evaluation confirming diagnosis of narcolepsy
- Xenazine
- Edited to require a documented contraindication or allergic reaction to generic tetrabenazine before brand name Xenazine will be approved
- Brand can cost up to ~$30,000 per month compared to generic cost of ~$18,840 per month
- Edited to require a documented contraindication or allergic reaction to generic tetrabenazine before brand name Xenazine will be approved
- Lyrica
- Added exclusion criteria to exclude coverage of Lyrica for trigeminal neuralgia due to lack of recommendation in the AAN and NICE guidelines
- Recommendations for treatment of trigeminal neuralgia include carbamazepine, oxcarbazepine, baclofen, lamotrigine, and pimozide
- Added exclusion criteria to exclude coverage of Lyrica for trigeminal neuralgia due to lack of recommendation in the AAN and NICE guidelines
- Lemtrada and Tysabri policies
- Current policies for both Lemtrada and Tysabri require a documented failure, intolerance, or contraindication to 2 or more unspecified disease modifying therapies for MS
- Ocrevus was FDA approved in March 2017 for Relapsing forms of Multiple Sclerosis (RMS) and Primary Progressive Multiple Sclerosis (PPMS)
- First drug approved for PPMS
- At approximately $6,500 per month, Ocrevus is the least costly of the disease-modifying therapies for MS
- Due to Ocrevus’ cost-saving opportunities, recommend updating Lemtrada and Tysabri policies to require documented failure, intolerance, or contraindication to Ocrevus and one additional unspecified disease modifying therapy
- Trokendi XR and Qudexy XR policies
- Established criteria for extended-release topiramate products since they received FDA approval for migraine prevention in April 2017
- Criteria for seizure diagnosis remains the same – 90-day trial with topiramate immediate release
- Diagnosis of migraine will require claims history illustrating 90-days use of immediate release topiramate in a 120-day period and 90-days use of a second migraine prophylactic in a 120-day period
- Second preventive drug must have an evidence rating of Level A or Level B by the American Academy of Neurology (AAN)
- Botox for Chronic Migraine Prevention
- Removed psychiatric evaluation requirement
- Removed abortive therapy requirement to better align with other insurers and to allow for use of over-the-counter treatments
- Updated prophylactic requirement to require claims review showing 90-days use within 120-day periods of 2 preventive drugs
- Preventive drugs must have evidence ratings of Level A or Level B by the AAN
- Dysport
- Dysport has received FDA indications for Adult Lower Limb Spasticity and Adult Upper Limb Spasticity
- Added same criteria as for Botox
- Recent new FDA indication for Pediatric Lower Limb Spasticity in children age 2 to 17 with Cerebral Palsy
- Dysport has received FDA indications for Adult Lower Limb Spasticity and Adult Upper Limb Spasticity
- H.P. Acthar
- H.P. Acthar gel is FDA indicated for Infantile Spasms and Multiple Sclerosis, but we only cover the drug for Infantile Spams
- We updated the exclusion criteria to specifically speak to the reasons why we don’t cover it for other uses
- Provigil and Nuvigil policy
Criteria Changes – Effective October 4, 2017
- Forteo
- Updated policy with language for Tymlos
- Orfadin and Nityr
- Added Nityr to what had been Orfadin policy
- Nityr is currently specialty pharmacy Tier 6 with PA
- IVIG
- Added Cuvitru, Hizentra, and Hyqvia to policy
- Added criteria for treatment of PANS/PANDAS
- Otezla
- Removed Humira and Enbrel ST
- Smoking Cessation
- Specified 1 quit attempt covered under wellness benefit per 180 days
- Edited bupropion criteria
- Edited Chantix criteria
- Defined medical necessity
Criteria Change – Effective January 1, 2018
- Opioids, Long-Acting and Short-Acting
- Added MED requirements
- Changed from brand name LA Opioids to non-preferred LA Opioids
- Added requirements to LA Opioid, Tramadol ER, and Nucynta sections
New Policies
- Triptan Managed Dose Limit
- Established the criteria for coverage of additional quantities of triptans above the established managed dose limit
- Vyvanse Chewable
- Tier 3 with PA
- Covered for members ages 6 to 12 years
- Quantity limit of #30 per 30 days
- Oncology Regimen Review – eviCore
- Offers guidance on the eviCore preauthorization review process for oncology agents and oncology supportive care agents
- Update: Dr. Belgrave recommended adding stem cell procedures to eviCore review exclusions
New Policy – Effective October 4, 2017
- Hereditary Angioedema (HAE)
- Replaced individual drug policies for Cinryze, Berinert, Firazyr, Kalbitor, and Ruconest
- Established criteria for coverage of formulary addition Haegarda
New Policy – Effective January 1, 2018
- Wellness Coverage for Statin Medications
- Establishes the criteria for coverage of atorvastatin, lovastatin, pravastatin, and simvastatin under the wellness benefit
Tier Changes
- Zubsolv – Moved from Excluded to Tier 3 with PA
- Pricing similar to Suboxone
- Recommending coverage to give providers more options
- Bunavail – Moved from Excluded to Tier 3 with PA
- Pricing similar to Suboxone
- Recommending coverage to give providers more options
- Evekeo (amphetamine) – Moved from Tier 3 to Excluded
- Multiple amphetamine products covered at Tier 1
- Conzip (tramadol) – Moved from Tier 3 to Excluded
- Tramadol is covered at Tier 1
- Zipsor (diclofenac) – Moved from Tier 3 to Excluded
- Multiple diclofenac products covered at Tier 1
- Pennsaid – Moved from Tier 3 to Excluded
- Diclofenac gel 1% covered at Tier 1
- Ziagen tablets – Moved from Tier 2 to Tier 3
- Abacavir covered at Tier 1
- Epzicom – Moved from Tier 2 to Tier 3
- Abacavir/lamivudine covered at Tier 1
- Videx EC – Moved from Tier 2 to Tier 3
- Didanosine covered at Tier 1
- Viramune – Moved from Tier 2 to Tier 3
- Nevirapine covered at Tier 1
- Viramune XR – Moved from Tier 2 to Tier 3
- Nevirapine ER covered at Tier 1
- Antabuse – Moved from Tier 2 to Tier 3
- Disulfiram covered at Tier 1
- Namenda – Moved from Tier 2 to Tier 3
- Memantine covered at Tier 1
- Emend Oral – Moved from Tier 2 to Tier 3
- Aprepitant is covered at Tier 1
- Avodart – Move from Tier 2 to Tier 3
- Dutasteride is covered at Tier 1
- Jalyn – Moved from Tier 2 to Tier 3
- Dutasteride/tamsulosin is covered at Tier 1
- Uroxatrol – Moved from Tier 2 to Tier 3
- Alfuzosin HCl ER is covered at Tier 1
- Moved these seizure disorder medications from Tier 2 to Tier 3, as all have generics at Tier 1:
- Carbatrol
- Depakene
- Depakote, Depakote Sprinkles
- Dilantin
- Gabitril
- Klonopin
- Lamictal, Lamictal XR
- Mysoline
- Neurontin
- Tegretol, Tegretol XR
- Topamax
- Zarontin
- Zonegran
- Note: DAW penalty will not be assessed if member chooses to remain on brand name instead of switching to generic
- Cafergot – Moved from Tier 2 to Tier 3
- Ergotamine/caffeine is covered at Tier 1
- Arixtra – Moved from Tier 2 to Tier 3
- Fondaparinux is covered at Tier 1
Tier Change – Effective October 4, 2017
- Sovaldi – Move from Tier 4 with PA to Tier 6 with PA
- Preferred products are Harvoni, Epclusa, and Mavyret
- Currently, there are no commercial members on Sovaldi