Reid October Newsletter
October 19, 2017CMS Education on Medicare Skilled Nursing and Therapy Services Coverage
The Centers for Medicare and Medicaid (CMS) want to remind you that the Jimmo v. Sebelius Settlement Agreement clarified that Medicare covers skilled nursing care and skilled therapy services under its skilled nursing facility, home health, and outpatient therapy benefits when a patient needs skilled care in order to maintain function or to prevent or slow decline or deterioration (provided all other coverage criteria are met). This may reflect a change in practice for those of you who thought that Medicare only covers nursing and therapy services under these benefits when a patient is expected to improve.
Specifically, this settlement required manual revisions to restate a maintenance coverage standard for both skilled nursing and therapy services under these benefits:
- Skilled nursing services would be covered where such services are necessary to maintain the patient’s current condition or prevent or slow further deterioration, so long as the patient requires skilled care for the services to be safely and effectively provided.
- Skilled therapy services are covered when an individualized assessment of the patient’s clinical condition demonstrates that the specialized judgment, knowledge, and skills of a qualified therapist are necessary for the performance of a safe and effective maintenance program. This type of maintenance program to maintain the patient’s current condition or to prevent or slow further deterioration is covered so long as the patient requires skilled care for the safe and effective performance of the program.
This decision does not:
- Mandate that you use daily services over intermittent skilled services for maintenance therapy.
- Override benefit limits. Benefit limits are still in place for certain services, which may prevent indefinite treatment in some situations.
- Require observation and assessment by a nurse for the treatment of the illness or injury when the characteristics are part of a longstanding pattern of a waxing and waning condition, which by themselves don’t require skilled services, and when there’s no attempt to change the treatment to resolve them. (A3-3132.1.C.2, SNF-214.1.C.2)
As part of our educational efforts to make sure services are provided and coverage determinations are adjudicated accurately and in accordance with existing Medicare policy, we ask that you review this educational information from CMS:
- New clarified summary
- CMS Manual Updates to Clarify Skilled Nursing Facility (SNF), Inpatient Rehabilitation Facility (IRF), Home Health (HH), and Outpatient (OPT) Coverage Pursuant to Jimmo vs. Sebelius
- MLN Matters on Manual Updates to Clarify Skilled Nursing Facility (SNF), Inpatient Rehabilitation Facility (IRF), Home Health (HH), and Outpatient (OPT) Coverage Pursuant to Jimmo vs. Sebelius
- Jimmo v. Sebelius Settlement Agreement Fact Sheet
- Jimmo v. Sebelius Settlement Agreement Program Manual Clarifications Fact Sheet
- PowerPoint from the National Call for Contractors and Adjudicators – December 16, 2013
eviCore Online Clinical Consultation Scheduling
eviCore is excited to introduce their online clinical consultation scheduling system to help streamline the process for requesting peer-to-peer consultations.
You can schedule clinical consultations for our members’ cases faster on eviCore.com. You can also find this from the eviCore.com provider login page, beneath the log in button.

Note: You can’t access this feature from Your Health Alliance for providers, you must go to eviCore.com. You will have to continue to go to Your Health Alliance for providers to submit eviCore preauthorization request.
From there, choose Health Alliance Med Plan from the Select Health Plan dropdown and select your solution.
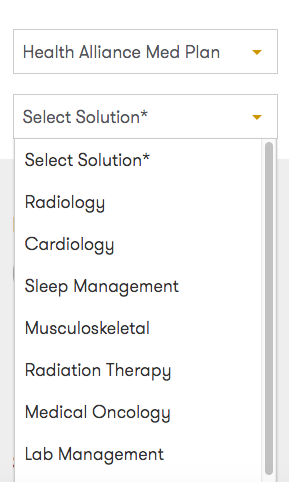
Fill out the form with the required info, and submit. Then you’ll receive a confirmation email, and an agent will contact you before the time in your request to schedule your exact appointment time.
Oncology Preauthorizations on eviCore
For which oncology patients is preauthorization on eviCore through Your Health Alliance for providers required? We can help:
| Patient Type | When Is PA Required? |
|---|---|
| Patients on stand-alone medications who have approvals on file with us for medications that required PA before August 1, 2017 | When the current authorization expires, or if treatment or medication changes. |
| Patients on standalone medications or regimens that did not require PA before August 1, 2017 | Required as of August 1, 2017. |
| Patients on stand-alone medications or regimens who have an approval on file with us for |
When the current authorization expires. We recommend these requests be placed through eviCore if the provider group would like an authorization verifying the full regimen. |
Reminder About In-Network Referrals
Remember to use contracted in-network providers before referring a patient outside our network. You can search a member’s network on Your Health Alliance for providers and office personnel by attaching to that member.
When our members need services that aren’t available from an in-network provider, they might also be able to get those services from a provider in their secondary or tertiary network. You can also access this while attached to a member. Note: All providers in secondary or tertiary networks require preauthorization.
Pharmacy Updates
All Plans
Formulary Additions
- Kevzara (sarilumab) – Indicated for the treatment of adult patients with moderate to severe Rheumatoid Arthritis (RA) who have had an inadequate response or intolerance to one or more biologic or non-biologic Disease-Modifying Anti-Rheumatic Drugs (DMARDs).
- Medicare – Non-formulary
- Austedo (deutetrabenazine) – Indicated for the treatment of chorea associated with Huntington’s disease (HD).
- Medicare – Non-formulary
- Ingrezza (valbenazine) – Indicated for the treatment of tardive dyskinesia (TD).
- Medicare – Tier 5 with PA
- Ocrevus (ocrelizumab) – Indicated for the treatment of relapsing or primary progressive forms of multiple sclerosis (MS) in adult patients.
- Medicare – Tier 5 with PA
- Radicava (edaravone) – Indicated for the treatment of Amyotrophic Lateral Sclerosis (ALS).
- Medicare – Non-formulary
- Xadago (safinamide) – Indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes.
- Medicare – Non-formulary
Formulary Additions – Effective October 4, 2017
- Brineura (cerliponase alfa) – Indicated to slow the loss of ambulation in symptomatic pediatric patients 3 years of age and older with late infantile neuronal ceroid lipofuscinosis type 2 (CLN2), also known as tripeptidyl peptidase 1 (TPP1) deficiency.
- Medicare – Part B only
- Emflaza (deflazacort) – Indicated for the treatment of Duchenne muscular dystrophy in patients 5 years of age and older.
- Medicare – Non-formulary
- Spinraza (nusinersen) – Indicated for the treatment of spinal muscular atrophy (SMA).
- Medicare – Part B only
- Tremfya (guselkumab) – Indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
- Medicare – Non-formulary
- Tymlos (abaloparatide) – Indicated for the treatment of postmenopausal women with osteoporosis at high risk for fracture defined as a history of osteoporotic fracture, multiple risk factors for fracture, or patients who have failed or are intolerant to other available osteoporosis therapy.
- Commercial – Tier 5 with PA
- Medicare – Non-formulary
- Haegarda (C1 inhibitor [human]) – Indicated as routine prophylaxis against angioedema attacks in adults and adolescents with hereditary angioedema.
- Medicare – Tier 6 with PA
- Nityr (nitisinone) – Indicated for the treatment of hereditary tyrosinemia type 1.
- Medicare – Non-formulary
- Hepatitis C
- Mavyret (glecaprevir and pibrentasvir) – Indicated for the treatment of chronic hepatitis C virus genotype 1, 2, 3, 4, 5, or 6 infection in adults without cirrhosis or with compensated cirrhosis, HCV genotype 1 infection in adults previously treated with a regimen containing an HCV NS5A inhibitor, or an NS3/4A protease inhibitor, but not both.
- Medicare – Tier 5 with PA
- Vosevi (sofosbuvir, velpatasvir, and voxilaprevir) – Indicated for the treatment of adults with chronic hepatitis C virus (HCV) infection, without cirrhosis or with compensated cirrhosis (Child-Pugh class A), who have genotype 1, 2, 3, 4, 5, or 6 infection, and have previously been treated with an HCV regimen containing an NS5A inhibitor, or who have genotype 1a or 3 infection and have previously been treated with an HCV regimen containing sofosbuvir without an NS5A inhibitor.
- Medicare – Non-formulary
- Mavyret (glecaprevir and pibrentasvir) – Indicated for the treatment of chronic hepatitis C virus genotype 1, 2, 3, 4, 5, or 6 infection in adults without cirrhosis or with compensated cirrhosis, HCV genotype 1 infection in adults previously treated with a regimen containing an HCV NS5A inhibitor, or an NS3/4A protease inhibitor, but not both.
- Oncology
- Kisqali (ribociclib) 200mg tablet – Indicated for breast cancer, advanced or metastatic.
- Medicare – Tier 5 with PA reviewed by Health Alliance
- Nerlynx (neratinib) 40mg tablet – Indicated for breast cancer.
- Medicare – Tier 5 with PA reviewed by Health Alliance
- Bavencio (avelumab) 200mg/10mL IV – Indicated for Merkel cell carcinoma, metastatic, and urothelial carcinoma, locally advanced or metastatic.
- Medicare – Tier 5 with PA, reviewed by Health Alliance if covered under Part D, reviewed by eviCore if covered under Part B
- Rydapt (midostaurin) 25mg capsule – Indicated for acute myeloid leukemia, FLT3-positive, mast cell leukemia, and systemic mastocytosis.
- Medicare – Tier 5 with PA reviewed by Health Alliance
- Alunbrig (brigatinib) 30mg tablet – Indicated for non-small cell lung cancer, metastatic.
- Medicare – Tier 5 with PA reviewed by Health Alliance
- Imfinzi (durvalumab) 120mg/2.4mL, 500mg/10mL IV – Urothelial carcinoma, locally advanced or metastatic.
- Medicare – Tier 5 with PA, reviewed by Health Alliance if covered under Part D, reviewed by eviCore if covered under Part B
- Kisqali (ribociclib) 200mg tablet – Indicated for breast cancer, advanced or metastatic.